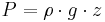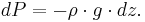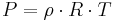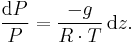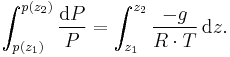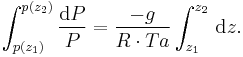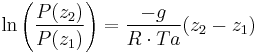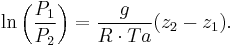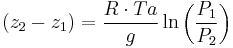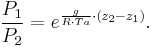Hypsometric equation
The hypsometric equation relates an atmospheric pressure ratio to the equivalent thickness of an atmospheric layer under the assumptions of constant temperature and gravity. It is derived from the hydrostatic equation and the ideal gas law.
It is expressed as:
where:
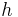 = thickness of the layer [m]
= thickness of the layer [m]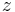 = geopotential height [m]
= geopotential height [m]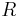 = gas constant for dry air
= gas constant for dry air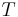 = temperature in kelvins [K]
= temperature in kelvins [K]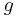 = gravitational acceleration [m/s2]
= gravitational acceleration [m/s2]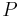 = pressure [Pa]
= pressure [Pa]
In meteorology 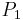 and
and 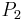 are isobaric surfaces and T is the average temperature of the layer between them. In altimetry with the International Standard Atmosphere the hypsometric equation is used to compute pressure at a given geopotential height in isothermal layers in the upper and lower stratosphere.
are isobaric surfaces and T is the average temperature of the layer between them. In altimetry with the International Standard Atmosphere the hypsometric equation is used to compute pressure at a given geopotential height in isothermal layers in the upper and lower stratosphere.
Derivation
The hydrostatic equation:
where 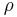 is the density [kg/m3], is used to generate the equation for hydrostatic equilibrium, written in differential form:
is the density [kg/m3], is used to generate the equation for hydrostatic equilibrium, written in differential form:
This is combined with the ideal gas law:
to eliminate 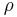 :
:
This is integrated from 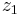 to
to 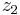 :
:
Where Ta is equal to the average column temperature.
Integration gives:
simplifying to:
Rearranging:
or, eliminating the logarithm:
![\ h = z_2 - z_1 = \frac{R \cdot T}{g} \cdot \ln \left [ \frac{P_1}{P_2} \right ]](/2012-wikipedia_en_all_nopic_01_2012/I/fd291c9dac8360c7ea00f1afadc66154.png)